
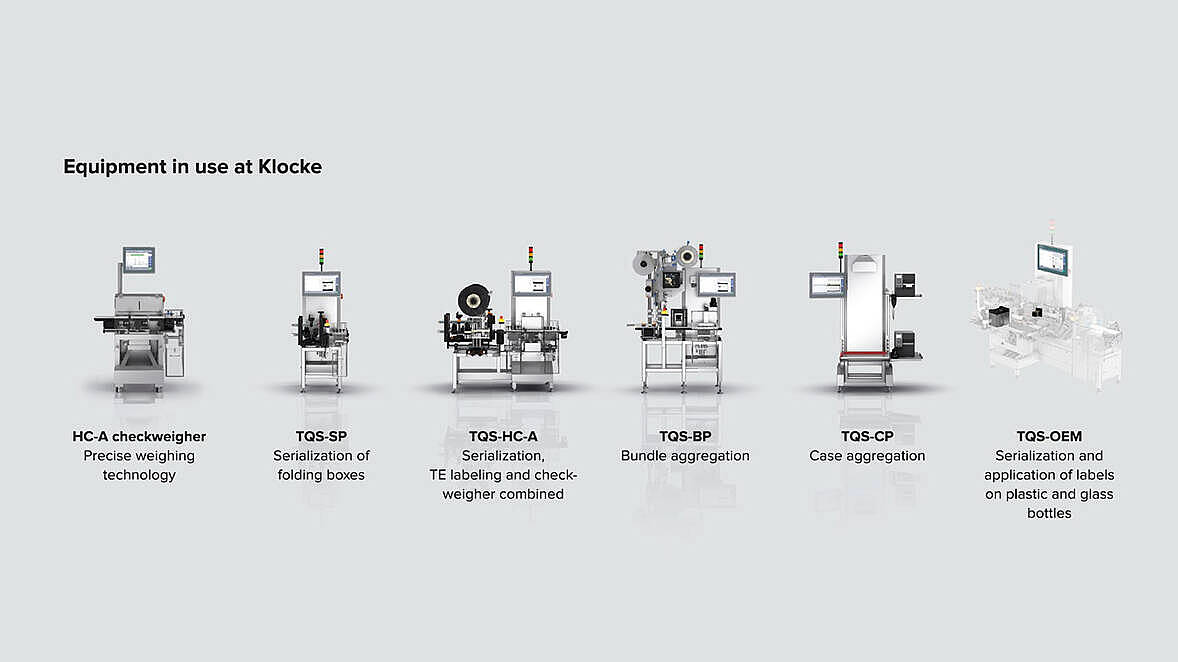
Klocke Group’s packaging lines require the highest quality standards. A wide range of products such as cosmetics, technology and medical products are packaged in these lines – but the main focus is on pharmaceuticals in typical blister packs and plastic or glass bottles. Marking requirements are becoming more complex and vary depending on the market they are to be sold in. As a result, obligations for serialization and aggregation are also becoming more complex in many sectors. On top of that, there are customer-specific needs to address. The Klocke Group has shown foresight in its pioneering role and made the necessary adaptations early on. Every line at the packaging site has appropriate TQS solutions from Wipotec that reliably comply with all regulations and enable full redundancy. This makes frequent format changeovers quick, easy and smooth.
Starting signal for serialization
To provide its customers with the best possible quality, the Klocke Group invests in the best technology. When the first regulations on serialization were introduced, the contract manufacturer reacted early and looked for future-proof solutions. “In retrospect, this fast response time proved to be a considerable advantage for us,” recalls Dominik Katarzynski, Managing Director of the Weingarten site.
“We used the time before implementation of the regulations effectively to select suitable machines.” Systems have to be compact, user-friendly and accurate, so that manual format changeovers can be easily implemented. Wipotec’s systems were impressive right from the start – but the actual added value became evident in the production process.
“Our positive first impression was confirmed.”
Dominik Katarzynski, Managing Director
Products and packaging types
In over 60 years of company history, the Klocke Group has become one of Europe’s leading contract manufacturers and packers in the pharmaceutical sector. “We have explicitly designed all our packaging lines for primary and secondary packaging to meet our needs. These are quick format changeovers and a compact design.” Klocke’s activities fall into three categories:
1. Pharmaceutical products in solid dosage forms make up the majority of the core business. When operating at full capacity, the 20 packaging lines can produce 500 million blister packs a year.
2. The second area is veterinary pharmaceuticals: “Even without serialization regulations, many of our customers now expect a data matrix code on the packaging.”
3. In the area of special packaging, the focus is on technical products or cosmetics, among others, whose marking usually only requires batch coding. But also, medical devices that are labeled with UDI are part of this category.
Unique Features
As a contract manufacturer, the Klocke Group relies on fully redundant lines to be able to process all products at all stations: “That is why every line is equipped with TQS units. We would not have hesitated to invest in different systems, had we not been as satisfied as we were after installing the first systems. “
Serialization without limits
Keeping a steady focus on the future, the Klocke Group wanted to think ahead from the outset and cover all serialization requirements and aggregation levels. Wipotec’s complete solutions were the right choice to achieve this: “We initially equipped one line with the complete equipment and then rolled it out to all other packaging lines.” TQS has proven to be exceptionally flexible, particularly when having to adapt to new standards, such as the most recent change to UDI for medical devices.
Frequent format changeovers
The employees working on the lines have to change formats often due to the diverse product portfolio. Changeover times therefore are a key factor for operational efficiency. Hardware and software must adapt to new items as quickly and easily as possible in a user-friendly way. This is the great advantage of the TQS units: “Without Wipotec, we would not be able to work as efficiently as we do. We can excellently perform article changes at our TQS stations. The intuitive operation of the machines is an enormous help to achieve this,” explains Katarzynski. Compared to other solutions tested, he estimates the associated time savings to be up to 40 percent.
“The most significant advantage of Wipotec technology for us is that we are able to do everything with it.”
Dominik Katarzynski, Managing Director
All-round service
Time is precious in production. The clock is already ticking during commissioning: “The installation went perfectly. We were ready for serialization in half an hour thanks to plug and play.” Klocke’s order books are full, so production has to keep running. Planned maintenance and updates, as well as spontaneous service calls, must not compromise system operational readiness. That’s another reason why Klocke values speedy support which is available either remotely or on site: “I receive excellent feedback about Wipotec’s service from colleagues on the line. This has also enabled us to correct errors, which has saved us additional costs and rework.
Together into the future
TQS has convinced all along the line. The Klocke Group is highly satisfied: “Even special requests by our customers – such as printing data matrix codes linking to a website – can be implemented at lightning speed with Wipotec systems.” “This adaptability is crucial for us as a contract manufacturer. We are currently seeing a huge number of new inquiries,” reports Katarzynski. “This means that we will implement additional lines and, of course, continue to count on Wipotec systems.” Several new TQS units where delivered and are ready to be deployed.
“We have now installed the 50th Wipotec unit – I think that says it all.”
Dominik Katarzynski, Managing Director
Subscribe to our Blog
Sign up for our Blog Newsletter and be notified only if new articles are published.
