Falsified medicines directives – preventing drug falsification
Drug safety is a valuable commodity that is repeatedly put at risk by falsified drugs. Falsified drugs can conceal medicines with incorrectly declared, diluted or missing ingredients. Retailers and patients are misled by deceptively genuine packaging or repackaging in original boxes.
Counterfeits are usually manufactured and sold much more cheaply than the real drugs. According to an estimate by the US FDA (Food and Drug Administration), more than 10% of all drugs in circulation are believed to be counterfeit. The possible implications of this are that patients believe they are dealing with the correct drugs but are unknowingly taking ineffective or even harmful substances, with potentially drastic consequences for their health.
Honest pharmaceutical producers not only have to accept lost sales due to the falsified cheap competition but also loss of confidence among consumers who feel deceived. The falsified medicines directives required by the World Health Organisation and introduced by many countries are aimed at safeguarding patients’ health. Therefore, achieving regulatory compliance is in the interest of all reputable pharmaceutical producers.
Need more information about global falsified medicines directives? We are happy to advise you!
We inform you on this page about the global falsified medicines directives:
Falsified medicines directives worldwide – an overview
Overview of falsification guidelines worldwide
Legislators all over the world have resolutely opposed the dangers of falsified drugs for consumers and companies by adopting national and supranational falsification directives. Compliance with the regulations enables pharmaceutical manufacturers and packaging service providers to ensure that their products can be distributed seamlessly in the target markets and falsified low-cost products are reliably removed from those markets. There are multiple falsification directives worldwide at various stages of implementation, with partly differing requirements.
In this overview, you will find out where the similarities and peculiarities lie.
- Coding
- Tamper-Evident seal
- Aggregation
- GS1 international
Coding: Other countries, other labelling of medicines
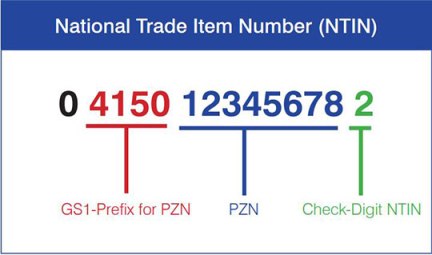
All falsification directives require an individual product identification number on the drug packaging. In addition, most of the directives include the expiry date for the drug as well as a batch number and possibly a serial number. The format of these labels can vary widely, but they must include human-readable plain text as well as a machine-readable barcode. The same applies to the type of product identification number – the GTIN format of the GS1 is often required, sometimes an ID number in the NTIN or in country-specific formats is specified (e.g. PPN in Germany, CIP in France, KDC in Korea, CNK in Belgium, AIC in Italy, etc.). The product identification number usually contains the following information: A country prefix, the company number, article number and a check digit.


Tamper-Evident seal: Special feature of the EU Falsified Medicines Directive
The tamper-evident seal is required for prescription-only drugs in countries which are subject to the EU Falsified Medicines Directive that entered into force on February 9, 2019. The seal prevents subsequent manipulation of the packaged medicine or the concealment of falsified drugs in original packaging. The product must not be dispensed to the customer if the seal is damaged or opened when sold.
The solution for the tamper-proof sealing of drug boxes:


Aggregation: Track & Trace throughout the complete supply chain
Only some directives, e.g. in Turkey, require an aggregation that allows the traceability of a single product pack along the entire transport route and across several packaging levels. Countries such as the USA still reserve the right to make this decision, in the EU and some other countries, aggregation is voluntary. In Brazil, it must be possible to trace every single box across the various aggregation levels from 2021. Here too, the type of coding may vary depending on the country. Although in some falsification directives – like the European one – aggregation is voluntary from a purely legal point of view, it may nevertheless be desired by customers such as wholesalers and hospital pharmacies. They accept what are sometimes large deliveries in pack or pallet size, where the ability to verify a complete delivery straightaway at goods receipt considerably facilitates their flow of goods.
With our aggregations solutions TQS-CP and TQS-CP Bottle this is exactly what you’re providing your customers with: Drugs that are verifiable at pack and pallet level. The operator fills the shipping box in layers. The integrated camera captures the codes of the medicine boxes after each layer. When the box is filled and the aggregation level is complete, the TQS-CP automatically creates the correct label. This works on multiple aggregation levels.
GS1 international – guardian of the identification numbers
Based in Brussels, GS1 international designs global standards for a variety of industries and allocates the Global Trade Item Numbers (GTIN) and other identification numbers for drugs. It guarantees international communication via standardised barcodes and identification numbers. Thanks to the close cooperation between Wipotec, GS1 Germany and the GS1 Global Healthcare User Group, we quickly pass on innovations to our customers and implement relevant requirements immediately.

Europe’s falsified medicines directive 2011/62/EU and what it means for manufacturers in the EU
In 2011, the EU Falsified Medicines Directive 2011/62/EU was published. It applies to all prescription-only and selected OTC drugs in the European Union since February 2019. The non-EU countries Iceland and Norway are also subject to the European Falsified Medicines Directive. Italy, Belgium and Greece serialised even before February 2019. Not in accordance with the EU directive, but using vignettes or bollini (stickers). They have a further six years in which to implement the EU directive in full. Drugs which do not bear the necessary security features according to the EU FMD (Falsified Medicines Directive) may no longer be sold in the EU. In practice, the pharmacist will scan the individual pack before dispensing it to the patient. The drug must not be dispensed if the scan returns an error message.
Need more information about the EU-FMD?

Details of EU Directive 2011/62/EU
According to the FMD, each single package must contain the following information in plain text and encoded in a machine-readable 2D matrix code:
- Product identification number, in the format GTIN, NTIN (or PPN for Germany)
- Batch number
- Batch number
- Individual serial number of the single pack
- If necessary, national healthcare reimbursement numbers, NHRN, for reimbursement by national healthcare insurers
In addition, single packs must be protected against manipulation with an intact tamper-evident seal.
securPharm: National verification system
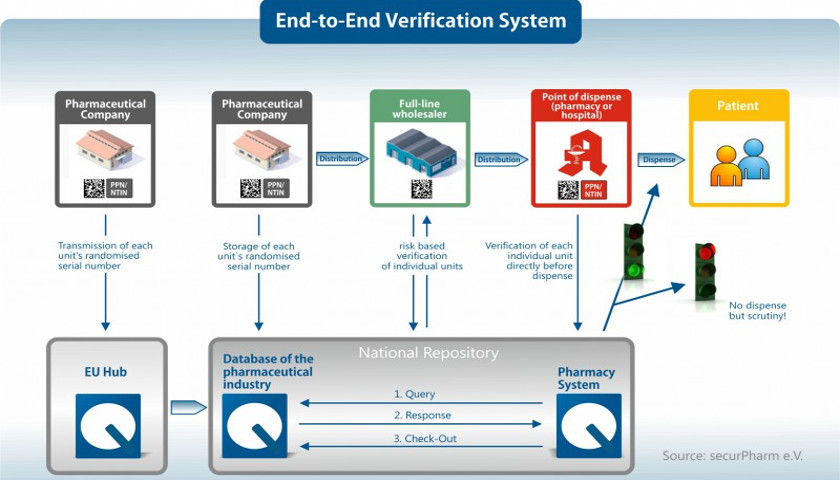
securPharm: National verification system guarantees safe medicines in Germany
As soon as a drug package is scanned at the pharmacy, the scanned code is matched with an online database to verify that it is a legal, safe medicine. The European countries have each set up national systems in the respective national language in which the corresponding data are stored. In Germany, the system operates under the name securPharm. The national standard identification Pharmazentralnummer (PZN), usually encoded in a barcode, which was previously common in Germany, is included in the National Trade Item Number NTIN or Pharmacy-Product-Number PPN prescribed by the European Falsification Prevention Directive when introduced by securPharm. It is recorded together with the other required information in the 2D matrix code.
European Medicines Verification Organisation (EMVO)
Identifying drugs EU-wide with the help of the EMVO
Since 2015, the national databases have been grouped together in a European hub. This ensures comparability of the data throughout Europe, thus enabling internal European trade in safe pharmaceutical products. This EU hub is under the supervision of the European Medicines Verification Organisation, EMVO for short, which is continuing to develop and operate the project. Manufacturers of pharmaceutical products feed the required data directly into the European database, which in turn communicates with the national databases. The establishment of national and international databases was completed by 2019, in time for the entry into force of the European Falsification Directive.
DSCSA – the Drug Supply Chain Security Act ensures safe medicines in the USA
The US falsification directive DCSA is part of the FDA’s DQSA (Drug Quality Security Act). It was signed by the President in 2013 and is expected to be fully implemented by 2023. Since 2015, manufacturers and retailers of prescription-only and reimbursable drugs have been obligated to provide the necessary information at batch level and to record all transaction data until the medicine is finally dispensed in the pharmacy. Complete traceability of the batch numbers of medicines makes it possible to quickly recognise and seize not only falsified but also stolen batches of drugs. In this case, staggered deadlines apply to the various stakeholders, producers, packaging service providers, wholesalers and vendors. From November 2023, the obligation will be extended to the labelling of medicines in single packages. The transitional period for the labelling of single packages starts from November 2018. Corresponding specifications previously applied in individual states and are now being replaced by the US-wide regulation.
Need more information about the DSCSA? Request further information or consultation now!
This information is required by the DSCSA
From November 2018, the following information must be shown on every individual pack in the form of a 2D matrix code:
- National product identification number in GTIN format
- Individual serial number of the single package
- Drug expiry date
- Batch name of the medicine
Serialisation of HDPE bottles
Serialisation of HDPE bottles in the USA:
Manufacturers and packers for the US market are faced with another challenge besides the implementation of the DSCSA directive: the HDPE bottles customary in the USA for tablets and capsules. In contrast to the folding boxes with blister packs common in Europe, they make very different demands on the manufacturer’s logistics. This also includes the so-called helper codes. They are applied on the lid or base of the bottles to aggregate the bottles into larger bundles. This can also be done “invisibly” with UV ink. The helper code enables identification of the packs, even if the individual bottle labels are no longer legible due to bundling.
The bottle series from Wipotec reliably prints and inspects bottles and vials. Find your solution to product inspection, reliable serialisation and semi-automatic aggregation of bottles and vials to meet the requirements of the American falsification directive DCSA.
Ultra-fast all-round inspection – compact serialisation – semi-automatic aggregation – printing of helper codes
Drug labelling with e-pedigree
US-wide traceability of drug labelling with e-pedigree
To a certain extent, e-pedigree is the American counterpart to the European EMV system. All data of a preparation are stored in this “electronic pedigree”, including the transaction data which are collected along the supply chain of a pharmaceutical product. Here, too, there are smaller-scale state-level databases that are grouped together in an international database. Many of the federal state databases are based on the pioneering Florida system that introduced e-pedigree as early as 2005.
Counterfeiting directive in South Africa: Serialisation of secondary and teritary packaging
Falsified drugs directive in Brazil:
Track & Trace with SNCM
The Brazilian health authority ANVISA is a step ahead. In 2014, it adopted the SNCM’s abridged regulations for a Track & Trace system used to monitor manufacturing and the supply chain of drugs. Similarly to the European and American systems, the product identification number, serial number, batch number and expiry date of the drugs should be printed on the packaging. After some delays in the implementation, the regulation currently applicable specifies 1 April 2022 as the deadline for comprehensive implementation of the “Sistema Nacional de Controle de Medicamentos”.
Need more information about global falsification directives?

South Africa has joined the global fight against counterfeit medicines. Pharmaceutical exports from the EU to South Africa exceed €1 billion a year, making the recent coding and serialisation directives in South Africa a major change for European pharmaceutical manufacturers. Companies shipping to South Africa will have to reorganise their packaging processes to meet the requirements. The serialisation of secondary and tertiary packaging is expected to be completed by 2022. The gradual implementation of the regulation aims to give pharmaceutical companies sufficient time to prepare.
Implementation of the falsified medicines directives – how do packers and pharmaceutical professionals accomplish this?
The implementation of national and international falsification directives poses major challenges to pharmaceutical manufacturers and packaging service providers, both of a technical nature and with regard to the mindset within the company. Wipotec can help you to overcome these obstacles with tailor-made solutions and a wealth of information. Above all, the requirement to label not only every batch but every single pack individually, often necessitates changes in the production line and new purchases. Manufacturers and packers therefore face a number of difficulties. Manufacturers and packers therefore face a number of challenges.
Find out how Wipotec helps you to overcome these challenges.
Action at management level
Achieving regulatory compliance requires effective cooperation
In reality, we find that in many companies the changeover in the production line is more than “just” a technical problem that the production manager can manage single-handedly. It is often necessary to set up meetings with several solution providers in order to find a perfect solution that suits both the requirements of the target countries and also the conditions within their own company. Contract manufacturers need to contact their clients and partners in good time because it often takes time to find the best solution. The challenge is to recognise the extremely high priority of these issues and to allow sufficient time to clarify them. Several tasks have to be tackled simultaneously – not just variable solutions for coding and labelling packages and batches, but also data processing and data transmission to national databases.
This is where the solutions from Wipotec offer a significant advantage: thanks to the open interfaces of our machines, they are compatible with the software solutions of several level 3 service providers.
Find out more about the advantages of the open XML interfaces!
Can’t wait any longer? TQS Fast Track is the fast plug-and-play solution for fully automated serialisation within 6 weeks!
Print quality and data management
Print quality and data management requirements necessitate investment
The capital expenditure incurred for finding, procuring and integrating the appropriate solutions should also not be underestimated. The printing of packages with long-lasting, high-resolution codes and labels that are always machine readable is no small challenge. The high speeds involved make it necessary to use suitable product transport technologies to avoid smudging the print. Light-fastness and abrasion resistance of the print has to be guaranteed on different surfaces to guarantee the codes remain readable during the product’s entire lifetime. Only with a high degree of consistency is it possible to prevent the image recognition systems from rejecting products. Particularly for small codes with high density, appropriate printing and product transport systems are essential to ensure the necessary marking quality at high printing speeds.
To ensure compliance with the falsification directives, pharmaceutical manufacturers and packaging service providers should not make false economies. The ROI is continued marketability.
Flexibility
An international market requires a high degree of flexibility
Manufacturers who supply several regions from one plant need to have the necessary flexibility for different formats and print layouts. This also means that the packaging line and its printer units can be changed over to the relevant falsification directives quickly and accurately. For this, manufacturers have to create the individual data set for each individual product and then download it to the relevant national or international database system. The easy-to-operate user interfaces of the solutions from Wipotec with the ConfigureFast software function enable country-specific codes to be set quickly and accurately without any delays in operation. The flexible software makes changing codes straightforward. The integrated LineManager initially stores all production data in the local cache and transfers it to the connected level 3 system. The temporary memory protects your data against loss in the event of power outages and other unpredictable events.
Compact solutions where space is tight: Track & Trace on the Fast Track
Free space is often in short supply in the manufacturing and packaging lines of pharmaceutical manufacturers and contract packagers The very compact Wipotec machines make it easy to integrate important functions in existing lines with a minimum of space. The best part of this: with our Fast Track solutions, your serialisation will be up and running within only 6 weeks.
The TQS-SP enables the serialisation and tamper-evident labelling of individual drug packs in the smallest of spaces. With the TQS-HC-A with Tamper-Evident you can perform three tasks fully automatically on a length of 1800 m: checkweighing, serialising and labelling.
Your benefits:
- Reliable compliance with international falsification directives
- Even where space is at a premium
- Solution within six weeks of ordering
- Modular, scalable and easily integrated in existing lines
Find out now about the most compact serialisation solutions for the EU market!

Single Pack Serialisation

Serialisation | Tamper-Evident | Weighing
Why it’s worth focusing on chances, not challenges
It’s worth seeing the international falsification directives as an opportunity to optimise your business performance and contribute to patient protection.
- Full compliance with the FMD will guarantee your continued marketability.
- You’ll be one step ahead of your competitors due to your flexibility with our solutions.
- The traceability of products required by the falsification directives will provide you with valuable Track & Trace data on the efficiency of your supply chain and sales – allowing you to control and optimise your operations.
Find the right solution for your application now!
Successful solutions for the pharmaceutical industry: Find out more about customer projects with Wipotec.